recount3 quick start guide
Leonardo Collado-Torres
Lieber Institute for Brain Development, Johns Hopkins Medical CampusCenter for Computational Biology, Johns Hopkins Universitylcolladotor@gmail.com
24 September 2025
Source:vignettes/recount3-quickstart.Rmd
recount3-quickstart.RmdOverview
The recount3 R/Bioconductor package is an interface to the recount3 project. recount3 provides uniformly processed RNA-seq data for hundreds of thousands of samples. The R package makes it possible to easily retrieve this data in standard Bioconductor containers, including RangedSummarizedExperiment. The sections on terminology and available data contains more detail on those subjects.
The main documentation website for all the
recount3-related projects is available at recount.bio.
Please check that website for more information about how this
R/Bioconductor package and other tools are related to each other.
Basics
Installing recount3
R is an open-source statistical environment which can be
easily modified to enhance its functionality via packages. recount3
is a R package available via the Bioconductor repository
for packages. R can be installed on any operating system
from CRAN after which you can
install recount3
by using the following commands in your R session:
if (!requireNamespace("BiocManager", quietly = TRUE)) {
install.packages("BiocManager")
}
BiocManager::install("recount3")
## Check that you have a valid Bioconductor installation
BiocManager::valid()You can install the development version from GitHub with:
BiocManager::install("LieberInstitute/recount3")Required knowledge
recount3 is based on many other packages and in particular in those that have implemented the infrastructure needed for dealing with RNA-seq data. A recount3 user will benefit from being familiar with SummarizedExperiment to understand the objects recount3 generates. It might also prove to be highly beneficial to check the
- DESeq2 and limma packages for performing differential expression analysis with the RangedSummarizedExperiment objects,
- DEFormats package for switching the objects to those used by other differential expression packages such as edgeR,
- derfinder package for performing annotation-agnostic differential expression analysis.
-
recount
package which provided an R/Bioconductor interface to the data in the
recount2project (Collado-Torres, Nellore, Kammers, Ellis et al., 2017; Collado-Torres, Nellore, and Jaffe, 2017).
If you are asking yourself the question “Where do I start using Bioconductor?” you might be interested in this blog post.
Asking for help
As package developers, we try to explain clearly how to use our
packages and in which order to use the functions. But R and
Bioconductor have a steep learning curve so it is critical
to learn where to ask for help. The blog post quoted above mentions some
but we would like to highlight the Bioconductor support site
as the main resource for getting help: remember to use the
recount3 tag and check the older
posts. Other alternatives are available such as creating GitHub
issues and tweeting. However, please note that if you want to receive
help you should adhere to the posting
guidelines. It is particularly critical that you provide a small
reproducible example and your session information so package developers
can track down the source of the error.
Citing recount3
We hope that recount3 will be useful for your research. Please use the following information to cite the package and the overall approach. Thank you!
## Citation info
citation("recount3")
#> To cite package 'recount3' in publications use:
#>
#> Collado-Torres L (2025). _Explore and download data from the recount3
#> project_. doi:10.18129/B9.bioc.recount3
#> <https://doi.org/10.18129/B9.bioc.recount3>,
#> https://github.com/LieberInstitute/recount3 - R package version
#> 1.19.3, <http://www.bioconductor.org/packages/recount3>.
#>
#> Wilks C, Zheng SC, Chen FY, Charles R, Solomon B, Ling JP, Imada EL,
#> Zhang D, Joseph L, Leek JT, Jaffe AE, Nellore A, Collado-Torres L,
#> Hansen KD, Langmead B (2021). "recount3: summaries and queries for
#> large-scale RNA-seq expression and splicing." _Genome Biol_.
#> doi:10.1186/s13059-021-02533-6
#> <https://doi.org/10.1186/s13059-021-02533-6>,
#> <https://doi.org/10.1186/s13059-021-02533-6>.
#>
#> To see these entries in BibTeX format, use 'print(<citation>,
#> bibtex=TRUE)', 'toBibtex(.)', or set
#> 'options(citation.bibtex.max=999)'.Quick start
After installing recount3 (Wilks, Zheng, Chen, Charles et al., 2021), we need to load the package, which will automatically load the required dependencies.
## Load recount3 R package
library("recount3")If you have identified a study of interest and want
to access the gene level expression data, use create_rse()
as shown below. create_rse() has arguments that will allow
you to specify the annotation of interest for the given
organism, and whether you want to download gene,
exon or exon-exon junction expression
data.
## Find all available human projects
human_projects <- available_projects()
#> 2025-09-24 22:49:53.699545 caching file sra.recount_project.MD.gz.
#> 2025-09-24 22:49:54.446132 caching file gtex.recount_project.MD.gz.
#> 2025-09-24 22:49:55.135577 caching file tcga.recount_project.MD.gz.
## Find the project you are interested in,
## here we use SRP009615 as an example
proj_info <- subset(
human_projects,
project == "SRP009615" & project_type == "data_sources"
)
## Create a RangedSummarizedExperiment (RSE) object at the gene level
rse_gene_SRP009615 <- create_rse(proj_info)
#> 2025-09-24 22:49:59.02621 downloading and reading the metadata.
#> 2025-09-24 22:49:59.752585 caching file sra.sra.SRP009615.MD.gz.
#> 2025-09-24 22:50:00.73781 caching file sra.recount_project.SRP009615.MD.gz.
#> 2025-09-24 22:50:01.548428 caching file sra.recount_qc.SRP009615.MD.gz.
#> 2025-09-24 22:50:02.55013 caching file sra.recount_seq_qc.SRP009615.MD.gz.
#> 2025-09-24 22:50:03.487661 caching file sra.recount_pred.SRP009615.MD.gz.
#> 2025-09-24 22:50:03.547547 downloading and reading the feature information.
#> 2025-09-24 22:50:04.137337 caching file human.gene_sums.G026.gtf.gz.
#> 2025-09-24 22:50:04.525175 downloading and reading the counts: 12 samples across 63856 features.
#> 2025-09-24 22:50:05.339601 caching file sra.gene_sums.SRP009615.G026.gz.
#> 2025-09-24 22:50:05.522864 constructing the RangedSummarizedExperiment (rse) object.
## Explore that RSE object
rse_gene_SRP009615
#> class: RangedSummarizedExperiment
#> dim: 63856 12
#> metadata(8): time_created recount3_version ... annotation recount3_url
#> assays(1): raw_counts
#> rownames(63856): ENSG00000278704.1 ENSG00000277400.1 ...
#> ENSG00000182484.15_PAR_Y ENSG00000227159.8_PAR_Y
#> rowData names(10): source type ... havana_gene tag
#> colnames(12): SRR387777 SRR387778 ... SRR389077 SRR389078
#> colData names(175): rail_id external_id ...
#> recount_pred.curated.cell_line BigWigURLOnce you have a RSE file, you can use transform_counts()
to transform the raw coverage counts.
## Once you have your RSE object, you can transform the raw coverage
## base-pair coverage counts using transform_counts().
## For RPKM, TPM or read outputs, check the details in transform_counts().
assay(rse_gene_SRP009615, "counts") <- transform_counts(rse_gene_SRP009615)Now you are ready to continue with downstream analysis software.
recount3 also supports accessing the BigWig raw coverage files as well as specific study or collection sample metadata. Please continue to the users guide for more detailed information.
Users guide
recount3
(Wilks, Zheng,
Chen, Charles et al., 2021) provides an interface for downloading
the recount3
raw files and building Bioconductor-friendly R objects
(Huber,
Carey, Gentleman, Anders et al., 2015;
Morgan,
Obenchain, Hester, and Pagès, 2019) that can be used with many
downstream packages. To achieve this, the raw data is organized by
study from a specific data source.
That same study can be a part of one or more
collections, which is a manually curated set of studies
with collection-specific sample metadata (see the Data
source vs collection for details). To get started with recount3,
you will need to identify the ID for the study of interest from either
human or mouse for a particular
annotation of interest. Once you have identified study,
data source or collection, and annotation, recount3
can be used to build a RangedSummarizedExperiment object
(Morgan,
Obenchain, Hester, and Pagès, 2019) for either
gene, exon or exon-exon
junction expression feature data. Furthermore, recount3
provides access to the coverage BigWig files that can be quantified for
custom set of genomic regions using megadepth.
Furthermore, snapcount
allows fast-queries for custom exon-exon junctions and other custom
input.
Available data
recount3
provides access to most of the recount3
raw files in a form that is R/Bioconductor-friendly. As a summary of
the data provided by the recount3 project (Figure
@ref(fig:recountWorkflowFig1)), the main data files provided are:
-
metadata: information about the samples in
recount3, which can come from sources such as the Sequence Read Archive as well asrecount3quality metrics. -
gene: RNA expression data quantified at the gene
annotation level. This information is provided for multiple annotations
that are organism-specific. Similar to the
recount2project, therecount3project provides counts at the base-pair coverage level (Collado-Torres, Nellore, and Jaffe, 2017). - exon: RNA expression data quantified at the exon annotation level. The data is also annotation-specific and the counts are also at the base-pair coverage level.
- exon-exon junctions: RNA expression data quantified at the exon-exon junction level. This data is annotation-agnostic (it does not depend on the annotation) and is variable across each study because different sets of exon-exon junctions are measured in each study.
- bigWig: RNA expression data in raw format at the base-pair coverage resolution. This raw data when coupled with a given annotation can be used to generate gene and exon level counts using software such as megadepth. It enables exploring the RNA expression landscape in an annotation-agnostic way.
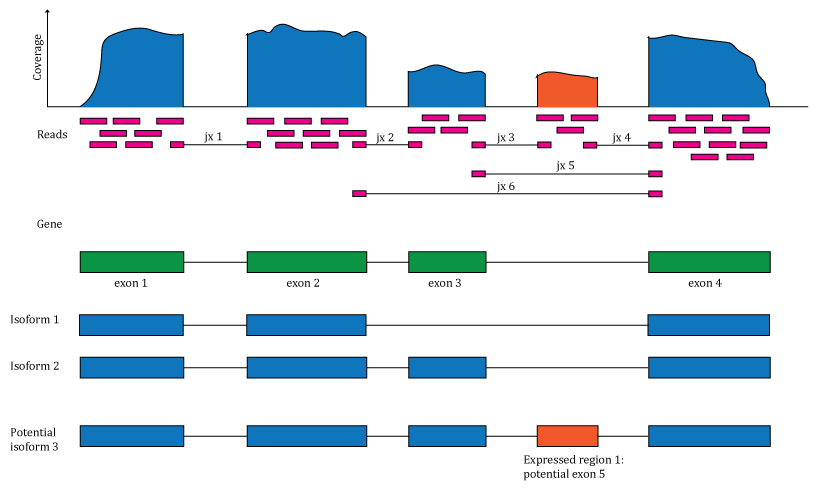
Overview of the data available in recount2 and recount3. Reads (pink boxes) aligned to the reference genome can be used to compute a base-pair coverage curve and identify exon-exon junctions (split reads). Gene and exon count matrices are generated using annotation information providing the gene (green boxes) and exon (blue boxes) coordinates together with the base-level coverage curve. The reads spanning exon-exon junctions (jx) are used to compute a third count matrix that might include unannotated junctions (jx 3 and 4). Without using annotation information, expressed regions (orange box) can be determined from the base-level coverage curve to then construct data-driven count matrices. DOI: < https://doi.org/10.12688/f1000research.12223.1>.
Terminology
Here we describe some of the common terminology and acronyms used
throughout the rest of the documentation. recount3
enables creating RangedSummarizedExperiment objects that
contain expression quantitative data (Figure
@ref(fig:recountWorkflowFig2)). As a quick overview, some of the main
terms are:
-
rse: a
RangedSummarizedExperimentobject from SummarizedExperiment (Morgan, Obenchain, Hester, and Pagès, 2019) that contains:-
counts: a matrix with the expression feature data
(either: gene, exon, or exon-exon junctions) and that can be accessed
using
assays(counts). -
metadata: a table with information about the
samples and quality metrics that can be accessed using
colData(rse). -
annotation: a table-like object with information
about the expression features which can be annotation-specific (gene and
exons) or annotation-agnostic (exon-exon junctions). This information
can be accessed using
rowRanges(rse).
-
counts: a matrix with the expression feature data
(either: gene, exon, or exon-exon junctions) and that can be accessed
using
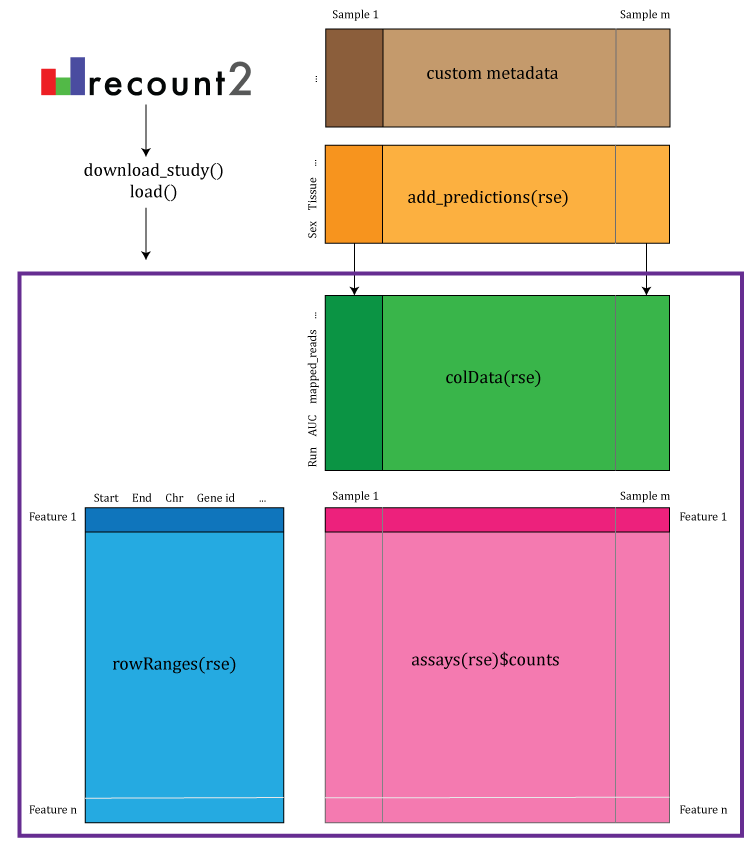
recount2 and recount3 provide coverage count matrices in
RangedSummarizedExperiment (rse) objects. Once the rse object has been
downloaded and loaded into R (using recount3::create_rse()
or recount2::download_study()), the feature information is
accessed with rowRanges(rse) (blue box), the counts with
assays(rse) (pink box) and the sample metadata with
colData(rse) (green box). The sample metadata stored in
colData(rse) can be expanded with custom code (brown box)
matching by a unique sample identifier such as the SRA Run ID. The rse
object is inside the purple box and matching data is highlighted in each
box. DOI: < https://doi.org/10.12688/f1000research.12223.1>.
recount3 enables accessing data from multiple reference organisms from public projects. To identify these projects, the key terms we use are:
- project: the project ID, which is typically the same ID that is used externally to identify that project in databases like the Sequence Read Archive 1
-
project_home: the location for where the project is
located at in the main
recount3data host: IDIES SciServer. We have two types of project homes:-
data_source: this identifies where the raw data was
downloaded from for creating
recount3. -
collection: this identifies a human-curated set of
projects or samples. The main difference compared to a
data_source is that a collection has
collection-specific metadata. For example, someone might have created a
table of information about samples in several projects by reading the
papers describing these studies as in
recount-brain(Razmara, Ellis, Sokolowski, Davis et al., 2019).
-
data_source: this identifies where the raw data was
downloaded from for creating
- organism: the reference organism (species) for the RNA-seq data, which is either human (hg38) or mouse (mm10).
Many of the recount3
raw files include three columns that are used to identify each
sample and that allow merging the data across these files. Those
are:
-
rail_id: an internal ID used by the
recountteam. The name stems fromRail-RNAwhich was the aligner used for generated the data inrecount2(Collado-Torres, Nellore, Kammers, Ellis et al., 2017). -
external_id: the external ID for the samle, such as
the Sequence Read Archive
runID. - study: the project ID.
Find a study
In order to access data from recount3, the first step is to identify a project that you are interested in working with. Most of the project IDs are the ones you can find on the Sequence Read Archive (SRA). For example, SRP009615 which we use in the examples in this vignette.The exceptions are the Genotype-Expression and The Cancer Genome Atlas human studies, commonly known as GTEx and TCGA. Both GTEx and TCGA are available in recount3 by tissue.
Through available_projects()
While you can use external websites to find a study of interest, you
can also use available_projects() to list the projects that
are available in recount3
as shown below. This will return a data.frame() object that
lists the unique project IDs.
human_projects <- available_projects()
#> 2025-09-24 22:50:06.422673 caching file sra.recount_project.MD.gz.
#> 2025-09-24 22:50:07.086169 caching file gtex.recount_project.MD.gz.
#> 2025-09-24 22:50:07.554508 caching file tcga.recount_project.MD.gz.
dim(human_projects)
#> [1] 8742 6
head(human_projects)
#> project organism file_source project_home project_type n_samples
#> 1 SRP107565 human sra data_sources/sra data_sources 216
#> 2 SRP149665 human sra data_sources/sra data_sources 4
#> 3 SRP017465 human sra data_sources/sra data_sources 23
#> 4 SRP119165 human sra data_sources/sra data_sources 6
#> 5 SRP133965 human sra data_sources/sra data_sources 12
#> 6 SRP096765 human sra data_sources/sra data_sources 7
## Select a study of interest
project_info <- subset(
human_projects,
project == "SRP009615" & project_type == "data_sources"
)
project_info
#> project organism file_source project_home project_type n_samples
#> 1838 SRP009615 human sra data_sources/sra data_sources 12Let’s say that you are interested in the GTEx projects, you could
then filter by file_source. We’ll focus only on those
entries that from a data source, and not from a collection for now.
subset(human_projects, file_source == "gtex" & project_type == "data_sources")
#> project organism file_source project_home project_type
#> 8678 ADIPOSE_TISSUE human gtex data_sources/gtex data_sources
#> 8679 MUSCLE human gtex data_sources/gtex data_sources
#> 8680 BLOOD_VESSEL human gtex data_sources/gtex data_sources
#> 8681 HEART human gtex data_sources/gtex data_sources
#> 8682 OVARY human gtex data_sources/gtex data_sources
#> 8683 UTERUS human gtex data_sources/gtex data_sources
#> 8684 VAGINA human gtex data_sources/gtex data_sources
#> 8685 BREAST human gtex data_sources/gtex data_sources
#> 8686 SKIN human gtex data_sources/gtex data_sources
#> 8687 SALIVARY_GLAND human gtex data_sources/gtex data_sources
#> 8688 BRAIN human gtex data_sources/gtex data_sources
#> 8689 ADRENAL_GLAND human gtex data_sources/gtex data_sources
#> 8690 THYROID human gtex data_sources/gtex data_sources
#> 8691 LUNG human gtex data_sources/gtex data_sources
#> 8692 SPLEEN human gtex data_sources/gtex data_sources
#> 8693 PANCREAS human gtex data_sources/gtex data_sources
#> 8694 ESOPHAGUS human gtex data_sources/gtex data_sources
#> 8695 STOMACH human gtex data_sources/gtex data_sources
#> 8696 COLON human gtex data_sources/gtex data_sources
#> 8697 SMALL_INTESTINE human gtex data_sources/gtex data_sources
#> 8698 PROSTATE human gtex data_sources/gtex data_sources
#> 8699 TESTIS human gtex data_sources/gtex data_sources
#> 8700 NERVE human gtex data_sources/gtex data_sources
#> 8701 PITUITARY human gtex data_sources/gtex data_sources
#> 8702 BLOOD human gtex data_sources/gtex data_sources
#> 8703 LIVER human gtex data_sources/gtex data_sources
#> 8704 KIDNEY human gtex data_sources/gtex data_sources
#> 8705 CERVIX_UTERI human gtex data_sources/gtex data_sources
#> 8706 FALLOPIAN_TUBE human gtex data_sources/gtex data_sources
#> 8707 BLADDER human gtex data_sources/gtex data_sources
#> 8708 STUDY_NA human gtex data_sources/gtex data_sources
#> 8709 BONE_MARROW human gtex data_sources/gtex data_sources
#> n_samples
#> 8678 1293
#> 8679 881
#> 8680 1398
#> 8681 942
#> 8682 195
#> 8683 159
#> 8684 173
#> 8685 482
#> 8686 1940
#> 8687 178
#> 8688 2931
#> 8689 274
#> 8690 706
#> 8691 655
#> 8692 255
#> 8693 360
#> 8694 1577
#> 8695 384
#> 8696 822
#> 8697 193
#> 8698 263
#> 8699 410
#> 8700 659
#> 8701 301
#> 8702 1048
#> 8703 251
#> 8704 98
#> 8705 19
#> 8706 9
#> 8707 21
#> 8708 133
#> 8709 204Note that one of the projects for GTEx is STUDY_NA,
that’s because in recount3
we processed all GTEx samples, including some that had no tissue
assigned and were not used by the GTEx consortium.
Ultimately, we need three pieces of information in order to download a specific dataset from recount3. Those are:
- project: the project ID,
- organism: either human or mouse,
- project_home: where the data lives which varies between data sources and collections.
project_info[, c("project", "organism", "project_home")]
#> project organism project_home
#> 1838 SRP009615 human data_sources/sraAvailable annotations
Now that we have identified our project of interest, the next step is
to choose an annotation that we want to work with. The annotation files
available depend on the organism. To facilitate finding the specific
names we use in recount3,
we have provided the function annotation_options().
annotation_options("human")
#> [1] "gencode_v26" "gencode_v29" "fantom6_cat" "refseq" "ercc"
#> [6] "sirv"
annotation_options("mouse")
#> [1] "gencode_v23"The main sources are:
- GENCODE project
- RefSeq: NCBI Reference Sequence Database
- FANTOM6_CAT which was first used with
recount2(Imada, Sanchez, Collado-Torres, Wilks et al., 2020) - ERCC: External RNA Controls Consortium 2, commercially available from ThermoFisher Scientific
- SIRV 3, commercially available from Lexogen
In recount3
we have provided multiple annotations, which is different from recount
(recount2 R/Bioconductor package) where all files were
computed using GENCODE version 25. However, in both, you might be
interested in quantifying your annotation of interest, as described
further below in the BigWig files section.
Build a RSE
Once you have chosen an annotation and a project, you can now build a
RangedSummarizedExperiment object
(Huber, Carey, Gentleman,
Anders et al., 2015;
Morgan,
Obenchain, Hester, and Pagès, 2019). To do so, we recommend using
the create_rse() function as shown below for GENCODE v26
(the default annotation for human files). create_rse()
internally uses several other recount3
functions for locating the necessary raw files, downloading them,
reading them, and building the RangedSummarizedExperiment
(RSE) object. create_rse() shows several status message
updates that you can silence with
suppressMessages(create_rse()) if you want to.
## Create a RSE object at the gene level
rse_gene_SRP009615 <- create_rse(project_info)
#> 2025-09-24 22:50:10.295059 downloading and reading the metadata.
#> 2025-09-24 22:50:11.007701 caching file sra.sra.SRP009615.MD.gz.
#> 2025-09-24 22:50:11.928057 caching file sra.recount_project.SRP009615.MD.gz.
#> 2025-09-24 22:50:12.904326 caching file sra.recount_qc.SRP009615.MD.gz.
#> 2025-09-24 22:50:13.80728 caching file sra.recount_seq_qc.SRP009615.MD.gz.
#> 2025-09-24 22:50:14.482621 caching file sra.recount_pred.SRP009615.MD.gz.
#> 2025-09-24 22:50:14.673431 downloading and reading the feature information.
#> 2025-09-24 22:50:15.353626 caching file human.gene_sums.G026.gtf.gz.
#> 2025-09-24 22:50:15.728374 downloading and reading the counts: 12 samples across 63856 features.
#> 2025-09-24 22:50:16.496029 caching file sra.gene_sums.SRP009615.G026.gz.
#> 2025-09-24 22:50:16.620635 constructing the RangedSummarizedExperiment (rse) object.
## Explore the resulting RSE gene object
rse_gene_SRP009615
#> class: RangedSummarizedExperiment
#> dim: 63856 12
#> metadata(8): time_created recount3_version ... annotation recount3_url
#> assays(1): raw_counts
#> rownames(63856): ENSG00000278704.1 ENSG00000277400.1 ...
#> ENSG00000182484.15_PAR_Y ENSG00000227159.8_PAR_Y
#> rowData names(10): source type ... havana_gene tag
#> colnames(12): SRR387777 SRR387778 ... SRR389077 SRR389078
#> colData names(175): rail_id external_id ...
#> recount_pred.curated.cell_line BigWigURLExplore the RSE
Because the RSE object is created at run-time in recount3,
unlike the static files provided by recount
for recount2, create_rse() stores information
about how this RSE object was made under metadata(). This
information is useful in case you share the RSE object and someone else
wants to be able to re-make the object with the latest data 4.
## Information about how this RSE object was made
metadata(rse_gene_SRP009615)
#> $time_created
#> [1] "2025-09-24 22:50:16 UTC"
#>
#> $recount3_version
#> package ondiskversion loadedversion path
#> recount3 recount3 1.19.3 1.19.3 /__w/_temp/Library/recount3
#> loadedpath attached is_base date source
#> recount3 /__w/_temp/Library/recount3 TRUE FALSE 2025-09-24 Bioconductor
#> md5ok library
#> recount3 NA /__w/_temp/Library
#>
#> $project
#> [1] "SRP009615"
#>
#> $project_home
#> [1] "data_sources/sra"
#>
#> $type
#> [1] "gene"
#>
#> $organism
#> [1] "human"
#>
#> $annotation
#> [1] "gencode_v26"
#>
#> $recount3_url
#> [1] "http://duffel.rail.bio/recount3"The SRP009615 study was composed of 12 samples, for which we have
63,856 genes in GENCODE v26. The annotation-specific information is
available through rowRanges() as shown below with the
gene_id column used to identify genes in each of the
annotations 5.
## Number of genes by number of samples
dim(rse_gene_SRP009615)
#> [1] 63856 12
## Information about the genes
rowRanges(rse_gene_SRP009615)
#> GRanges object with 63856 ranges and 10 metadata columns:
#> seqnames ranges strand | source
#> <Rle> <IRanges> <Rle> | <factor>
#> ENSG00000278704.1 GL000009.2 56140-58376 - | ENSEMBL
#> ENSG00000277400.1 GL000194.1 53590-115018 - | ENSEMBL
#> ENSG00000274847.1 GL000194.1 53594-115055 - | ENSEMBL
#> ENSG00000277428.1 GL000195.1 37434-37534 - | ENSEMBL
#> ENSG00000276256.1 GL000195.1 42939-49164 - | ENSEMBL
#> ... ... ... ... . ...
#> ENSG00000124334.17_PAR_Y chrY 57184101-57197337 + | HAVANA
#> ENSG00000185203.12_PAR_Y chrY 57201143-57203357 - | HAVANA
#> ENSG00000270726.6_PAR_Y chrY 57190738-57208756 + | HAVANA
#> ENSG00000182484.15_PAR_Y chrY 57207346-57212230 + | HAVANA
#> ENSG00000227159.8_PAR_Y chrY 57212184-57214397 - | HAVANA
#> type bp_length phase gene_id
#> <factor> <numeric> <integer> <character>
#> ENSG00000278704.1 gene 2237 <NA> ENSG00000278704.1
#> ENSG00000277400.1 gene 2179 <NA> ENSG00000277400.1
#> ENSG00000274847.1 gene 1599 <NA> ENSG00000274847.1
#> ENSG00000277428.1 gene 101 <NA> ENSG00000277428.1
#> ENSG00000276256.1 gene 2195 <NA> ENSG00000276256.1
#> ... ... ... ... ...
#> ENSG00000124334.17_PAR_Y gene 2504 <NA> ENSG00000124334.17_P..
#> ENSG00000185203.12_PAR_Y gene 1054 <NA> ENSG00000185203.12_P..
#> ENSG00000270726.6_PAR_Y gene 773 <NA> ENSG00000270726.6_PA..
#> ENSG00000182484.15_PAR_Y gene 4618 <NA> ENSG00000182484.15_P..
#> ENSG00000227159.8_PAR_Y gene 1306 <NA> ENSG00000227159.8_PA..
#> gene_type gene_name level
#> <character> <character> <character>
#> ENSG00000278704.1 protein_coding BX004987.1 3
#> ENSG00000277400.1 protein_coding AC145212.2 3
#> ENSG00000274847.1 protein_coding AC145212.1 3
#> ENSG00000277428.1 misc_RNA Y_RNA 3
#> ENSG00000276256.1 protein_coding AC011043.1 3
#> ... ... ... ...
#> ENSG00000124334.17_PAR_Y protein_coding IL9R 2
#> ENSG00000185203.12_PAR_Y antisense WASIR1 2
#> ENSG00000270726.6_PAR_Y processed_transcript AJ271736.10 2
#> ENSG00000182484.15_PAR_Y transcribed_unproces.. WASH6P 2
#> ENSG00000227159.8_PAR_Y unprocessed_pseudogene DDX11L16 2
#> havana_gene tag
#> <character> <character>
#> ENSG00000278704.1 <NA> <NA>
#> ENSG00000277400.1 <NA> <NA>
#> ENSG00000274847.1 <NA> <NA>
#> ENSG00000277428.1 <NA> <NA>
#> ENSG00000276256.1 <NA> <NA>
#> ... ... ...
#> ENSG00000124334.17_PAR_Y OTTHUMG00000022720.1 PAR
#> ENSG00000185203.12_PAR_Y OTTHUMG00000022676.3 PAR
#> ENSG00000270726.6_PAR_Y OTTHUMG00000184987.2 PAR
#> ENSG00000182484.15_PAR_Y OTTHUMG00000022677.5 PAR
#> ENSG00000227159.8_PAR_Y OTTHUMG00000022678.1 PAR
#> -------
#> seqinfo: 374 sequences from an unspecified genome; no seqlengthsSample metadata
The sample metadata provided in recount3
is much more extensive than the one in recount
for the recount2 project because it’s includes for quality
control metrics, predictions, and information used
internally by recount3
functions such as create_rse(). All individual metadata
tables include the columns rail_id,
external_id and study which are used
for merging the different tables. Finally, **BigWigUrl* provides the URL
for the BigWig file for the given sample.
## Sample metadata
recount3_cols <- colnames(colData(rse_gene_SRP009615))
## How many are there?
length(recount3_cols)
#> [1] 175
## View the first few ones
head(recount3_cols)
#> [1] "rail_id" "external_id" "study"
#> [4] "sra.sample_acc.x" "sra.experiment_acc" "sra.submission_acc"
## Group them by source
recount3_cols_groups <- table(gsub("\\..*", "", recount3_cols))
## Common sources and number of columns per source
recount3_cols_groups[recount3_cols_groups > 1]
#>
#> recount_pred recount_project recount_qc recount_seq_qc sra
#> 7 5 109 12 38
## Unique columns
recount3_cols_groups[recount3_cols_groups == 1]
#>
#> BigWigURL external_id rail_id study
#> 1 1 1 1
## Explore them all
recount3_colsFor studies from SRA, we can further extract the SRA attributes using
expand_sra_attributes() as shown below.
rse_gene_SRP009615_expanded <-
expand_sra_attributes(rse_gene_SRP009615)
colData(rse_gene_SRP009615_expanded)[, ncol(colData(rse_gene_SRP009615)):ncol(colData(rse_gene_SRP009615_expanded))]
#> DataFrame with 12 rows and 5 columns
#> BigWigURL sra_attribute.cells
#> <character> <character>
#> SRR387777 http://duffel.rail.b.. K562
#> SRR387778 http://duffel.rail.b.. K562
#> SRR387779 http://duffel.rail.b.. K562
#> SRR387780 http://duffel.rail.b.. K562
#> SRR389079 http://duffel.rail.b.. K562
#> ... ... ...
#> SRR389082 http://duffel.rail.b.. K562
#> SRR389083 http://duffel.rail.b.. K562
#> SRR389084 http://duffel.rail.b.. K562
#> SRR389077 http://duffel.rail.b.. K562
#> SRR389078 http://duffel.rail.b.. K562
#> sra_attribute.shRNA_expression sra_attribute.source_name
#> <character> <character>
#> SRR387777 no SL2933
#> SRR387778 yes, targeting SRF SL2934
#> SRR387779 no SL5265
#> SRR387780 yes targeting SRF SL3141
#> SRR389079 no shRNA expression SL6485
#> ... ... ...
#> SRR389082 expressing shRNA tar.. SL2592
#> SRR389083 no shRNA expression SL4337
#> SRR389084 expressing shRNA tar.. SL4326
#> SRR389077 no shRNA expression SL1584
#> SRR389078 expressing shRNA tar.. SL1583
#> sra_attribute.treatment
#> <character>
#> SRR387777 Puromycin
#> SRR387778 Puromycin, doxycycline
#> SRR387779 Puromycin
#> SRR387780 Puromycin, doxycycline
#> SRR389079 Puromycin
#> ... ...
#> SRR389082 Puromycin, doxycycline
#> SRR389083 Puromycin
#> SRR389084 Puromycin, doxycycline
#> SRR389077 Puromycin
#> SRR389078 Puromycin, doxycyclineCounts
The counts in recount3 are raw base-pair coverage counts, similar to those in recount. To further understand them, check the recountWorkflow DOI 10.12688/f1000research.12223.1. To highlight that these are raw base-pair coverage counts, they are stored in the “raw_counts” assay
assayNames(rse_gene_SRP009615)
#> [1] "raw_counts"Using transform_counts() you can scale the counts and
assign them to the “counts” assays slot to use them in downstream
packages such as DESeq2 and
limma.
## Once you have your RSE object, you can transform the raw coverage
## base-pair coverage counts using transform_counts().
## For RPKM, TPM or read outputs, check the details in transform_counts().
assay(rse_gene_SRP009615, "counts") <- transform_counts(rse_gene_SRP009615)Just like with recount
for recount2, you can transform the raw base-pair coverage
counts
(Collado-Torres,
Nellore, and Jaffe, 2017) to read counts with
compute_read_counts(), RPKM with
recount::getRPKM() or TPM values with
recount::getTPM(). Check transform_counts()
from recount3
for more details.
Exon
recount3
provides an interface to raw
files that go beyond gene counts, as well as other features you
might be interested in. For instance, you might want to study expression
at the exon expression level instead of
gene. To do so, use the type argument in
create_rse() as shown below.
## Create a RSE object at the exon level
rse_exon_SRP009615 <- create_rse(
proj_info,
type = "exon"
)
#> 2025-09-24 22:50:17.268085 downloading and reading the metadata.
#> 2025-09-24 22:50:18.042565 caching file sra.sra.SRP009615.MD.gz.
#> 2025-09-24 22:50:18.995759 caching file sra.recount_project.SRP009615.MD.gz.
#> 2025-09-24 22:50:19.980678 caching file sra.recount_qc.SRP009615.MD.gz.
#> 2025-09-24 22:50:20.792514 caching file sra.recount_seq_qc.SRP009615.MD.gz.
#> 2025-09-24 22:50:21.722049 caching file sra.recount_pred.SRP009615.MD.gz.
#> 2025-09-24 22:50:21.784463 downloading and reading the feature information.
#> 2025-09-24 22:50:22.659039 caching file human.exon_sums.G026.gtf.gz.
#> 2025-09-24 22:50:40.419933 downloading and reading the counts: 12 samples across 1299686 features.
#> 2025-09-24 22:50:41.036128 caching file sra.exon_sums.SRP009615.G026.gz.
#> 2025-09-24 22:50:43.151007 constructing the RangedSummarizedExperiment (rse) object.
## Explore the resulting RSE exon object
rse_exon_SRP009615
#> class: RangedSummarizedExperiment
#> dim: 1299686 12
#> metadata(8): time_created recount3_version ... annotation recount3_url
#> assays(1): raw_counts
#> rownames(1299686): GL000009.2|56140|58376|- GL000194.1|53594|54832|-
#> ... chrY|57213880|57213964|- chrY|57214350|57214397|-
#> rowData names(21): source type ... ont ccdsid
#> colnames(12): SRR387777 SRR387778 ... SRR389077 SRR389078
#> colData names(175): rail_id external_id ...
#> recount_pred.curated.cell_line BigWigURL
## Explore the object
dim(rse_exon_SRP009615)
#> [1] 1299686 12Each exon is shown in this output, so, you might have to filter the
exons of interest. Unlike in recount2, these are actual
exons and not disjoint exons 6.
## Exon annotation information
rowRanges(rse_exon_SRP009615)
#> GRanges object with 1299686 ranges and 21 metadata columns:
#> seqnames ranges strand | source
#> <Rle> <IRanges> <Rle> | <factor>
#> GL000009.2|56140|58376|- GL000009.2 56140-58376 - | ENSEMBL
#> GL000194.1|53594|54832|- GL000194.1 53594-54832 - | ENSEMBL
#> GL000194.1|55446|55676|- GL000194.1 55446-55676 - | ENSEMBL
#> GL000194.1|53590|55676|- GL000194.1 53590-55676 - | ENSEMBL
#> GL000194.1|112792|112850|- GL000194.1 112792-112850 - | ENSEMBL
#> ... ... ... ... . ...
#> chrY|57212184|57213125|- chrY 57212184-57213125 - | HAVANA
#> chrY|57213204|57213357|- chrY 57213204-57213357 - | HAVANA
#> chrY|57213526|57213602|- chrY 57213526-57213602 - | HAVANA
#> chrY|57213880|57213964|- chrY 57213880-57213964 - | HAVANA
#> chrY|57214350|57214397|- chrY 57214350-57214397 - | HAVANA
#> type bp_length phase
#> <factor> <numeric> <integer>
#> GL000009.2|56140|58376|- exon 2237 <NA>
#> GL000194.1|53594|54832|- exon 1239 <NA>
#> GL000194.1|55446|55676|- exon 231 <NA>
#> GL000194.1|53590|55676|- exon 2087 <NA>
#> GL000194.1|112792|112850|- exon 59 <NA>
#> ... ... ... ...
#> chrY|57212184|57213125|- exon 942 <NA>
#> chrY|57213204|57213357|- exon 154 <NA>
#> chrY|57213526|57213602|- exon 77 <NA>
#> chrY|57213880|57213964|- exon 85 <NA>
#> chrY|57214350|57214397|- exon 48 <NA>
#> gene_id transcript_id
#> <character> <character>
#> GL000009.2|56140|58376|- ENSG00000278704.1 ENST00000618686.1
#> GL000194.1|53594|54832|- ENSG00000274847.1 ENST00000400754.4
#> GL000194.1|55446|55676|- ENSG00000274847.1 ENST00000400754.4
#> GL000194.1|53590|55676|- ENSG00000277400.1 ENST00000613230.1
#> GL000194.1|112792|112850|- ENSG00000274847.1 ENST00000400754.4
#> ... ... ...
#> chrY|57212184|57213125|- ENSG00000227159.8_PA.. ENST00000507418.6_PA..
#> chrY|57213204|57213357|- ENSG00000227159.8_PA.. ENST00000507418.6_PA..
#> chrY|57213526|57213602|- ENSG00000227159.8_PA.. ENST00000507418.6_PA..
#> chrY|57213880|57213964|- ENSG00000227159.8_PA.. ENST00000507418.6_PA..
#> chrY|57214350|57214397|- ENSG00000227159.8_PA.. ENST00000507418.6_PA..
#> gene_type gene_name
#> <character> <character>
#> GL000009.2|56140|58376|- protein_coding BX004987.1
#> GL000194.1|53594|54832|- protein_coding AC145212.1
#> GL000194.1|55446|55676|- protein_coding AC145212.1
#> GL000194.1|53590|55676|- protein_coding AC145212.2
#> GL000194.1|112792|112850|- protein_coding AC145212.1
#> ... ... ...
#> chrY|57212184|57213125|- unprocessed_pseudogene DDX11L16
#> chrY|57213204|57213357|- unprocessed_pseudogene DDX11L16
#> chrY|57213526|57213602|- unprocessed_pseudogene DDX11L16
#> chrY|57213880|57213964|- unprocessed_pseudogene DDX11L16
#> chrY|57214350|57214397|- unprocessed_pseudogene DDX11L16
#> transcript_type transcript_name exon_number
#> <character> <character> <character>
#> GL000009.2|56140|58376|- protein_coding BX004987.1-201 1
#> GL000194.1|53594|54832|- protein_coding AC145212.1-201 4
#> GL000194.1|55446|55676|- protein_coding AC145212.1-201 3
#> GL000194.1|53590|55676|- protein_coding AC145212.2-201 3
#> GL000194.1|112792|112850|- protein_coding AC145212.1-201 2
#> ... ... ... ...
#> chrY|57212184|57213125|- unprocessed_pseudogene DDX11L16-001 5
#> chrY|57213204|57213357|- unprocessed_pseudogene DDX11L16-001 4
#> chrY|57213526|57213602|- unprocessed_pseudogene DDX11L16-001 3
#> chrY|57213880|57213964|- unprocessed_pseudogene DDX11L16-001 2
#> chrY|57214350|57214397|- unprocessed_pseudogene DDX11L16-001 1
#> exon_id level protein_id
#> <character> <character> <character>
#> GL000009.2|56140|58376|- ENSE00003753029.1 3 ENSP00000484918.1
#> GL000194.1|53594|54832|- ENSE00002218789.2 3 ENSP00000478910.1
#> GL000194.1|55446|55676|- ENSE00003714436.1 3 ENSP00000478910.1
#> GL000194.1|53590|55676|- ENSE00003723764.1 3 ENSP00000483280.1
#> GL000194.1|112792|112850|- ENSE00003713687.1 3 ENSP00000478910.1
#> ... ... ... ...
#> chrY|57212184|57213125|- ENSE00002023900.1 2 <NA>
#> chrY|57213204|57213357|- ENSE00002036959.1 2 <NA>
#> chrY|57213526|57213602|- ENSE00002021169.1 2 <NA>
#> chrY|57213880|57213964|- ENSE00002046926.1 2 <NA>
#> chrY|57214350|57214397|- ENSE00002072208.1 2 <NA>
#> transcript_support_level tag
#> <character> <character>
#> GL000009.2|56140|58376|- NA basic
#> GL000194.1|53594|54832|- 1 basic
#> GL000194.1|55446|55676|- 1 basic
#> GL000194.1|53590|55676|- 1 basic
#> GL000194.1|112792|112850|- 1 basic
#> ... ... ...
#> chrY|57212184|57213125|- NA PAR
#> chrY|57213204|57213357|- NA PAR
#> chrY|57213526|57213602|- NA PAR
#> chrY|57213880|57213964|- NA PAR
#> chrY|57214350|57214397|- NA PAR
#> recount_exon_id havana_gene
#> <character> <character>
#> GL000009.2|56140|58376|- GL000009.2|56140|583.. <NA>
#> GL000194.1|53594|54832|- GL000194.1|53594|548.. <NA>
#> GL000194.1|55446|55676|- GL000194.1|55446|556.. <NA>
#> GL000194.1|53590|55676|- GL000194.1|53590|556.. <NA>
#> GL000194.1|112792|112850|- GL000194.1|112792|11.. <NA>
#> ... ... ...
#> chrY|57212184|57213125|- chrY|57212184|572131.. OTTHUMG00000022678.1
#> chrY|57213204|57213357|- chrY|57213204|572133.. OTTHUMG00000022678.1
#> chrY|57213526|57213602|- chrY|57213526|572136.. OTTHUMG00000022678.1
#> chrY|57213880|57213964|- chrY|57213880|572139.. OTTHUMG00000022678.1
#> chrY|57214350|57214397|- chrY|57214350|572143.. OTTHUMG00000022678.1
#> havana_transcript ont ccdsid
#> <character> <character> <character>
#> GL000009.2|56140|58376|- <NA> <NA> <NA>
#> GL000194.1|53594|54832|- <NA> <NA> <NA>
#> GL000194.1|55446|55676|- <NA> <NA> <NA>
#> GL000194.1|53590|55676|- <NA> <NA> <NA>
#> GL000194.1|112792|112850|- <NA> <NA> <NA>
#> ... ... ... ...
#> chrY|57212184|57213125|- OTTHUMT00000058841.1 PGO:0000005 <NA>
#> chrY|57213204|57213357|- OTTHUMT00000058841.1 PGO:0000005 <NA>
#> chrY|57213526|57213602|- OTTHUMT00000058841.1 PGO:0000005 <NA>
#> chrY|57213880|57213964|- OTTHUMT00000058841.1 PGO:0000005 <NA>
#> chrY|57214350|57214397|- OTTHUMT00000058841.1 PGO:0000005 <NA>
#> -------
#> seqinfo: 374 sequences from an unspecified genome; no seqlengths
## Exon ids are repeated because a given exon can be part of
## more than one transcript
length(unique(rowRanges(rse_exon_SRP009615)$exon_id))
#> [1] 742049Because there are many more exons than genes, this type of analysis
uses more computational resources. Thus, for some large projects you
might need to use a high performance computing environment. To help you
proceed with caution, create_rse() shows how many features
and samples it’s trying to access. So if you get an out of memory error,
you’ll know why that happened.
## Check how much memory the gene and exon RSE objects use
pryr::object_size(rse_gene_SRP009615)
#> 30.94 MB
pryr::object_size(rse_exon_SRP009615)
#> 528.18 MBExon-exon junctions
In recount3 we have also provided the option to create RSE files for exon-exon junctions. Unlike the gene/exon RSE files, only the junctions present in a given project are included in the files, so you’ll have to be more careful when merging exon-exon junction RSE files. Furthermore, these are actual read counts and not raw base-pair counts. Given the sparsity of the data, the counts are provided using a sparse matrix object from Matrix. Thus exon-exon junction files can be less memory demanding than the exon RSE files.
## Create a RSE object at the exon-exon junction level
rse_jxn_SRP009615 <- create_rse(
proj_info,
type = "jxn"
)
#> 2025-09-24 22:50:47.570598 downloading and reading the metadata.
#> 2025-09-24 22:50:48.328273 caching file sra.sra.SRP009615.MD.gz.
#> 2025-09-24 22:50:48.960717 caching file sra.recount_project.SRP009615.MD.gz.
#> 2025-09-24 22:50:49.618007 caching file sra.recount_qc.SRP009615.MD.gz.
#> 2025-09-24 22:50:50.208907 caching file sra.recount_seq_qc.SRP009615.MD.gz.
#> 2025-09-24 22:50:51.107994 caching file sra.recount_pred.SRP009615.MD.gz.
#> 2025-09-24 22:50:51.16612 downloading and reading the feature information.
#> 2025-09-24 22:50:52.002604 caching file sra.junctions.SRP009615.ALL.RR.gz.
#> 2025-09-24 22:50:53.265101 downloading and reading the counts: 12 samples across 281448 features.
#> 2025-09-24 22:50:54.125621 caching file sra.junctions.SRP009615.ALL.MM.gz.
#> 2025-09-24 22:50:54.559936 matching exon-exon junction counts with the metadata.
#> 2025-09-24 22:50:55.567007 caching file sra.junctions.SRP009615.ALL.ID.gz.
#> 2025-09-24 22:50:55.645645 constructing the RangedSummarizedExperiment (rse) object.
## Explore the resulting RSE exon-exon junctions object
rse_jxn_SRP009615
#> class: RangedSummarizedExperiment
#> dim: 281448 12
#> metadata(9): time_created recount3_version ... jxn_format recount3_url
#> assays(1): counts
#> rownames(281448): chr1:11845-12009:+ chr1:12698-13220:+ ...
#> chrY:56848810-56851543:- chrY:56850515-56850921:+
#> rowData names(6): length annotated ... left_annotated right_annotated
#> colnames(12): SRR387777 SRR387778 ... SRR389077 SRR389078
#> colData names(175): rail_id external_id ...
#> recount_pred.curated.cell_line BigWigURL
dim(rse_jxn_SRP009615)
#> [1] 281448 12
## Exon-exon junction information
rowRanges(rse_jxn_SRP009615)
#> GRanges object with 281448 ranges and 6 metadata columns:
#> seqnames ranges strand | length
#> <Rle> <IRanges> <Rle> | <integer>
#> chr1:11845-12009:+ chr1 11845-12009 + | 165
#> chr1:12698-13220:+ chr1 12698-13220 + | 523
#> chr1:14696-185174:- chr1 14696-185174 - | 170479
#> chr1:14830-14969:- chr1 14830-14969 - | 140
#> chr1:14830-15020:- chr1 14830-15020 - | 191
#> ... ... ... ... . ...
#> chrY:56846131-56846553:+ chrY 56846131-56846553 + | 423
#> chrY:56846268-56846553:+ chrY 56846268-56846553 + | 286
#> chrY:56846486-56846553:+ chrY 56846486-56846553 + | 68
#> chrY:56848810-56851543:- chrY 56848810-56851543 - | 2734
#> chrY:56850515-56850921:+ chrY 56850515-56850921 + | 407
#> annotated left_motif right_motif
#> <integer> <character> <character>
#> chr1:11845-12009:+ 0 GT AG
#> chr1:12698-13220:+ 1 GT AG
#> chr1:14696-185174:- 0 CT AC
#> chr1:14830-14969:- 1 CT AC
#> chr1:14830-15020:- 0 CT AC
#> ... ... ... ...
#> chrY:56846131-56846553:+ 0 GT AG
#> chrY:56846268-56846553:+ 0 GT AG
#> chrY:56846486-56846553:+ 0 GT AG
#> chrY:56848810-56851543:- 0 CT AC
#> chrY:56850515-56850921:+ 0 GT AG
#> left_annotated right_annotated
#> <character> <character>
#> chr1:11845-12009:+ 0 aC19,sG19
#> chr1:12698-13220:+ aC19,gC19,gC24,gC25,.. aC19,cH38,gC19,gC24,..
#> chr1:14696-185174:- 0 0
#> chr1:14830-14969:- aC19,cH38,gC19,kG19,.. aC19,cH38,gC19,kG19,..
#> chr1:14830-15020:- aC19,cH38,gC19,kG19,.. 0
#> ... ... ...
#> chrY:56846131-56846553:+ 0 0
#> chrY:56846268-56846553:+ 0 0
#> chrY:56846486-56846553:+ 0 0
#> chrY:56848810-56851543:- 0 0
#> chrY:56850515-56850921:+ 0 0
#> -------
#> seqinfo: 97 sequences from an unspecified genome; no seqlengths
## Memory used
pryr::object_size(rse_jxn_SRP009615)
#> 60.30 MBBigWig files
Internally we used GenomicFeatures::exonicParts() when
processing all annotations in recount3
instead of GenomicRanges::disjoin() that was used in
recount2. We then re-assembled the counts for each
exon/gene to create the count files provided in recount3.
However, you might want to exclude the overlapping exonic parts from the
counts. If that’s the case or if you are interested in specific regions
of the hg38/mm10 genomes, you might want to
access the coverage BigWig files.
## BigWig file names
## The full URL is stored in BigWigUrl
basename(head(rse_gene_SRP009615$BigWigURL))
#> [1] "sra.base_sums.SRP009615_SRR387777.ALL.bw"
#> [2] "sra.base_sums.SRP009615_SRR387778.ALL.bw"
#> [3] "sra.base_sums.SRP009615_SRR387779.ALL.bw"
#> [4] "sra.base_sums.SRP009615_SRR387780.ALL.bw"
#> [5] "sra.base_sums.SRP009615_SRR389079.ALL.bw"
#> [6] "sra.base_sums.SRP009615_SRR389080.ALL.bw"These BigWig files can be accessed using
rtracklayer::import.bw() from R, or other tools such as bwtool that
we’ve used in the past 7. Using them, you can compute a coverage
matrix for a given set of regions.
One new software we developed is megadepth
for which we have provided an R/Bioconductor package interface. megadepth
is faster at accessing BigWig files and is the software we used
internally for generating the recount3 data. megadepth
provides convenient to use functions for quantifying a set of regions,
which might be of interest for co-expression analyses where double
counting exonic parts can be problematic.
You can also use derfinder and derfinderPlot if you are interested in visualizing the base-pair coverage data for a specific region using these BigWig coverage files.
Local files
recount3 depends on BiocFileCache (Shepherd and Morgan, 2025) for organizing the raw files and caching them, such that if you use the same file more than once, you only have to download it once. BiocFileCache will automatically update the file if it detects that the file has changed in the source
If you want to inspect which files you have downloaded or even delete
them, them you’ll want to use recount3_cache_files() and
recount3_cache_rm() as illustrated below.
## List the URLs of the recount3 data that you have downloaded
recount3_cache_files()
#> [1] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/sra.recount_project.MD.gz"
#> [2] "http://duffel.rail.bio/recount3/human/data_sources/gtex/metadata/gtex.recount_project.MD.gz"
#> [3] "http://duffel.rail.bio/recount3/human/data_sources/tcga/metadata/tcga.recount_project.MD.gz"
#> [4] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/15/SRP009615/sra.sra.SRP009615.MD.gz"
#> [5] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/15/SRP009615/sra.recount_project.SRP009615.MD.gz"
#> [6] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/15/SRP009615/sra.recount_qc.SRP009615.MD.gz"
#> [7] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/15/SRP009615/sra.recount_seq_qc.SRP009615.MD.gz"
#> [8] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/15/SRP009615/sra.recount_pred.SRP009615.MD.gz"
#> [9] "http://duffel.rail.bio/recount3/human/annotations/gene_sums/human.gene_sums.G026.gtf.gz"
#> [10] "http://duffel.rail.bio/recount3/human/data_sources/sra/gene_sums/15/SRP009615/sra.gene_sums.SRP009615.G026.gz"
#> [11] "http://duffel.rail.bio/recount3/human/annotations/exon_sums/human.exon_sums.G026.gtf.gz"
#> [12] "http://duffel.rail.bio/recount3/human/data_sources/sra/exon_sums/15/SRP009615/sra.exon_sums.SRP009615.G026.gz"
#> [13] "http://duffel.rail.bio/recount3/human/data_sources/sra/junctions/15/SRP009615/sra.junctions.SRP009615.ALL.RR.gz"
#> [14] "http://duffel.rail.bio/recount3/human/data_sources/sra/junctions/15/SRP009615/sra.junctions.SRP009615.ALL.MM.gz"
#> [15] "http://duffel.rail.bio/recount3/human/data_sources/sra/junctions/15/SRP009615/sra.junctions.SRP009615.ALL.ID.gz"
#> [16] "http://duffel.rail.bio/recount3/mouse/data_sources/sra/metadata/sra.recount_project.MD.gz"
#> [17] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/99/DRP000499/sra.sra.DRP000499.MD.gz"
#> [18] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/99/DRP000499/sra.recount_project.DRP000499.MD.gz"
#> [19] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/99/DRP000499/sra.recount_qc.DRP000499.MD.gz"
#> [20] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/99/DRP000499/sra.recount_seq_qc.DRP000499.MD.gz"
#> [21] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/99/DRP000499/sra.recount_pred.DRP000499.MD.gz"
#> [22] "http://duffel.rail.bio/recount3/human/data_sources/sra/gene_sums/99/DRP000499/sra.gene_sums.DRP000499.G026.gz"
#> [23] "http://duffel.rail.bio/recount3/human/annotations/gene_sums/human.gene_sums.G029.gtf.gz"
#> [24] "http://duffel.rail.bio/recount3/human/data_sources/sra/gene_sums/15/SRP009615/sra.gene_sums.SRP009615.G029.gz"
#> [25] "http://duffel.rail.bio/recount3/human/annotations/gene_sums/human.gene_sums.F006.gtf.gz"
#> [26] "http://duffel.rail.bio/recount3/human/data_sources/sra/gene_sums/15/SRP009615/sra.gene_sums.SRP009615.F006.gz"
#> [27] "http://duffel.rail.bio/recount3/human/annotations/gene_sums/human.gene_sums.R109.gtf.gz"
#> [28] "http://duffel.rail.bio/recount3/human/data_sources/sra/gene_sums/15/SRP009615/sra.gene_sums.SRP009615.R109.gz"
#> [29] "http://duffel.rail.bio/recount3/human/annotations/gene_sums/human.gene_sums.ERCC.gtf.gz"
#> [30] "http://duffel.rail.bio/recount3/human/data_sources/sra/gene_sums/15/SRP009615/sra.gene_sums.SRP009615.ERCC.gz"
#> [31] "http://duffel.rail.bio/recount3/human/annotations/gene_sums/human.gene_sums.SIRV.gtf.gz"
#> [32] "http://duffel.rail.bio/recount3/human/data_sources/sra/gene_sums/15/SRP009615/sra.gene_sums.SRP009615.SIRV.gz"
#> [33] "http://snaptron.cs.jhu.edu/data/temp/recount3/human/collections/geuvadis_smartseq/metadata/geuvadis_smartseq.recount_project.gz"
#> [34] "http://snaptron.cs.jhu.edu/data/temp/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.sra.ERP110066.MD.gz"
#> [35] "http://snaptron.cs.jhu.edu/data/temp/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.recount_project.ERP110066.MD.gz"
#> [36] "http://snaptron.cs.jhu.edu/data/temp/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.recount_qc.ERP110066.MD.gz"
#> [37] "http://snaptron.cs.jhu.edu/data/temp/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.recount_pred.ERP110066.MD.gz"
#> [38] "http://snaptron.cs.jhu.edu/data/temp/recount3/human/collections/geuvadis_smartseq/metadata/geuvadis_smartseq.custom.gz"
#> [39] "http://snaptron.cs.jhu.edu/data/temp/recount3/human/annotations/gene_sums/human.gene_sums.G026.gtf.gz"
#> [40] "http://snaptron.cs.jhu.edu/data/temp/recount3/human/data_sources/sra/gene_sums/66/ERP110066/sra.gene_sums.ERP110066.G026.gz"
#> [41] "http://duffel.rail.bio/recount3/mouse/data_sources/sra/metadata/67/DRP002367/sra.sra.DRP002367.MD.gz"
#> [42] "http://duffel.rail.bio/recount3/mouse/data_sources/sra/metadata/67/DRP002367/sra.recount_project.DRP002367.MD.gz"
#> [43] "http://duffel.rail.bio/recount3/mouse/data_sources/sra/metadata/67/DRP002367/sra.recount_qc.DRP002367.MD.gz"
#> [44] "http://duffel.rail.bio/recount3/mouse/data_sources/sra/metadata/67/DRP002367/sra.recount_seq_qc.DRP002367.MD.gz"
#> [45] "http://duffel.rail.bio/recount3/mouse/data_sources/sra/metadata/67/DRP002367/sra.recount_pred.DRP002367.MD.gz"
#> [46] "http://duffel.rail.bio/recount3/mouse/annotations/gene_sums/mouse.gene_sums.M023.gtf.gz"
#> [47] "http://duffel.rail.bio/recount3/mouse/data_sources/sra/gene_sums/67/DRP002367/sra.gene_sums.DRP002367.M023.gz"
#> [48] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.sra.ERP110066.MD.gz"
#> [49] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.recount_project.ERP110066.MD.gz"
#> [50] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.recount_qc.ERP110066.MD.gz"
#> [51] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.recount_seq_qc.ERP110066.MD.gz"
#> [52] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/66/ERP110066/sra.recount_pred.ERP110066.MD.gz"
#> [53] "http://duffel.rail.bio/recount3/human/data_sources/gtex/metadata/ER/BLADDER/gtex.gtex.BLADDER.MD.gz"
#> [54] "http://duffel.rail.bio/recount3/human/data_sources/gtex/metadata/ER/BLADDER/gtex.recount_project.BLADDER.MD.gz"
#> [55] "http://duffel.rail.bio/recount3/human/data_sources/gtex/metadata/ER/BLADDER/gtex.recount_qc.BLADDER.MD.gz"
#> [56] "http://duffel.rail.bio/recount3/human/data_sources/gtex/metadata/ER/BLADDER/gtex.recount_seq_qc.BLADDER.MD.gz"
#> [57] "https://idies.jhu.edu/recount3/data/human/data_sources/sra/metadata/sra.recount_project.MD.gz"
#> [58] "https://idies.jhu.edu/recount3/data/human/data_sources/gtex/metadata/gtex.recount_project.MD.gz"
#> [59] "https://idies.jhu.edu/recount3/data/human/data_sources/tcga/metadata/tcga.recount_project.MD.gz"
#> [60] "https://idies.jhu.edu/recount3/data/mouse/data_sources/sra/metadata/sra.recount_project.MD.gz"
#> [61] "https://data.idies.jhu.edu/recount3/data//human/data_sources/sra/metadata/sra.recount_project.MD.gz"
#> [62] "https://data.idies.jhu.edu/recount3/data//human/data_sources/gtex/metadata/gtex.recount_project.MD.gz"
#> [63] "https://data.idies.jhu.edu/recount3/data//human/data_sources/tcga/metadata/tcga.recount_project.MD.gz"
#> [64] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/67/SRP103067/sra.sra.SRP103067.MD.gz"
#> [65] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/67/SRP103067/sra.recount_project.SRP103067.MD.gz"
#> [66] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/67/SRP103067/sra.recount_qc.SRP103067.MD.gz"
#> [67] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/67/SRP103067/sra.recount_seq_qc.SRP103067.MD.gz"
#> [68] "http://duffel.rail.bio/recount3/human/data_sources/sra/metadata/67/SRP103067/sra.recount_pred.SRP103067.MD.gz"
## Delete the recount3 files from your cache
recount3_cache_rm()
## Check that no files are listed
recount3_cache_files()Your own mirror
recount3
functions such as create_rse() have a
recount3_url argument that can be changed to point to a
mirror or to a path in your computing system. This argument enables
using recount3
with data stored in other locations, or even generated using the same
processing pipeline that was used for recount3
but for your own/private data.
The main documentation website documents how the raw files should be
organized in your mirror or for your own data. You can inspect the
structure of the data by checking the internals of
locate_url() and locate_url_ann(). Both
functions can be used to get the full list of URLs. In addition, for a
given mirror, available_projects() will show the local data
sources and collections. Finally, file_retrieve() won’t
cache the data if it detects that the data already exists in the local
disk.
In particular, this functionality can be useful if you want to access
the data through Registry of Open Data on
AWS or at IDIES using SciServer Compute, which
are the two official mirrors for recount3 data.
Teams involved
The ReCount family involves the following teams:
- Ben Langmead’s lab at JHU Computer Science
- Kasper Daniel Hansen’s lab at JHBSPH Biostatistics Department
- Leonardo Collado-Torres and Andrew E. Jaffe from LIBD
- Abhinav Nellore’s lab at OHSU
- Jeff Leek’s lab at JHBSPH Biostatistics Deparment
- Data hosted by the Registry of Open Data on AWS and SciServer from IDIES at JHU through a load balancer called duffel.
Project history
To clarify the relationship between the R/Bioconductor packages and
the phases of ReCount
(Frazee,
Langmead, and Leek, 2011) please check the table below:
| Year | Phase | Main references | R/Bioconductor |
|---|---|---|---|
| 2011 | ReCount |
DOI: 10.1186/1471-2105-12-449 | none |
| 2017 | recount2 |
DOI: 10.1038/nbt.3838 10.12688/f1000research.12223.1 | recount |
| 2021 | recount3 |
DOI: 10.1186/s13059-021-02533-6 | recount3 |
Other related tools
The ReCount team has worked on several software
solutions and projects that complement each other and enable you to
re-use public RNA-seq data. Another Bioconductor package that you might
be highly interested in is snapcount,
which allows you to use the Snaptron web services. In
particular, snapcount
is best for queries over a particular subset of genes or intervals
across all or most of the samples in
recount2/Snaptron.
We remind you that the main documentation website
for all the recount3-related projects is available at recount.bio.
Please check that website for more information about how this
R/Bioconductor package and other tools are related to each other.
Thank you!
P.S. An alternative version of this vignette is available that was made using pkgdown.
Reproducibility
The recount3 package (Collado-Torres, 2025) was made possible thanks to:
- R (R Core Team, 2025)
- BiocFileCache (Shepherd and Morgan, 2025)
- BiocStyle (Oleś, 2025)
- biocthis
- covr (Hester, 2023)
- data.table (Barrett, Dowle, Srinivasan, Gorecki et al., 2025)
- GenomicRanges (Lawrence, Huber, Pagès, Aboyoun et al., 2013)
- httr (Wickham, 2023)
- knitcitations (Boettiger, 2021)
- knitr (Xie, 2014)
- Matrix (Bates, Maechler, and Jagan, 2025)
- pryr (Wickham, 2023)
- RefManageR (McLean, 2017)
- rmarkdown (Allaire, Xie, Dervieux, McPherson et al., 2024)
- rtracklayer (Lawrence, Gentleman, and Carey, 2009)
- R.utils (Bengtsson, 2025)
- S4Vectors (Pagès, Lawrence, and Aboyoun, 2025)
- sessioninfo (Wickham, Chang, Flight, Müller et al., 2025)
- SummarizedExperiment (Morgan, Obenchain, Hester, and Pagès, 2019)
- testthat (Wickham, 2011)
Code for creating the vignette
## Create the vignette
library("rmarkdown")
system.time(render("recount3-quickstart.Rmd", "BiocStyle::html_document"))
## Extract the R code
library("knitr")
knit("recount3-quickstart.Rmd", tangle = TRUE)
## Clean up
file.remove("quickstartRef.bib")
#> [1] TRUEDate the vignette was generated.
#> [1] "2025-09-24 22:50:57 UTC"Wallclock time spent generating the vignette.
#> Time difference of 1.245 minsR session information.
#> ─ Session info ───────────────────────────────────────────────────────────────────────────────────────────────────────
#> setting value
#> version R version 4.5.1 (2025-06-13)
#> os Ubuntu 24.04.3 LTS
#> system x86_64, linux-gnu
#> ui X11
#> language en
#> collate en_US.UTF-8
#> ctype en_US.UTF-8
#> tz UTC
#> date 2025-09-24
#> pandoc 3.8 @ /usr/bin/ (via rmarkdown)
#> quarto 1.7.32 @ /usr/local/bin/quarto
#>
#> ─ Packages ───────────────────────────────────────────────────────────────────────────────────────────────────────────
#> package * version date (UTC) lib source
#> abind 1.4-8 2024-09-12 [1] RSPM (R 4.5.0)
#> backports 1.5.0 2024-05-23 [1] RSPM (R 4.5.0)
#> bibtex 0.5.1 2023-01-26 [1] RSPM (R 4.5.0)
#> Biobase * 2.68.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> BiocFileCache 2.16.2 2025-08-27 [1] Bioconductor 3.21 (R 4.5.1)
#> BiocGenerics * 0.54.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> BiocIO 1.18.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> BiocManager 1.30.26 2025-06-05 [2] CRAN (R 4.5.1)
#> BiocParallel 1.42.2 2025-09-14 [1] Bioconductor 3.21 (R 4.5.1)
#> BiocStyle * 2.36.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> Biostrings 2.76.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> bit 4.6.0 2025-03-06 [1] RSPM (R 4.5.0)
#> bit64 4.6.0-1 2025-01-16 [1] RSPM (R 4.5.0)
#> bitops 1.0-9 2024-10-03 [1] RSPM (R 4.5.0)
#> blob 1.2.4 2023-03-17 [1] RSPM (R 4.5.0)
#> bookdown 0.44 2025-08-21 [1] RSPM (R 4.5.0)
#> bslib 0.9.0 2025-01-30 [2] RSPM (R 4.5.0)
#> cachem 1.1.0 2024-05-16 [2] RSPM (R 4.5.0)
#> cli 3.6.5 2025-04-23 [2] RSPM (R 4.5.0)
#> codetools 0.2-20 2024-03-31 [3] CRAN (R 4.5.1)
#> crayon 1.5.3 2024-06-20 [2] RSPM (R 4.5.0)
#> curl 7.0.0 2025-08-19 [2] RSPM (R 4.5.0)
#> data.table 1.17.8 2025-07-10 [1] RSPM (R 4.5.0)
#> DBI 1.2.3 2024-06-02 [1] RSPM (R 4.5.0)
#> dbplyr 2.5.1 2025-09-10 [1] RSPM (R 4.5.0)
#> DelayedArray 0.34.1 2025-04-17 [1] Bioconductor 3.21 (R 4.5.0)
#> desc 1.4.3 2023-12-10 [2] RSPM (R 4.5.0)
#> digest 0.6.37 2024-08-19 [2] RSPM (R 4.5.0)
#> dplyr 1.1.4 2023-11-17 [1] RSPM (R 4.5.0)
#> evaluate 1.0.5 2025-08-27 [2] RSPM (R 4.5.0)
#> fastmap 1.2.0 2024-05-15 [2] RSPM (R 4.5.0)
#> filelock 1.0.3 2023-12-11 [1] RSPM (R 4.5.0)
#> fs 1.6.6 2025-04-12 [2] RSPM (R 4.5.0)
#> generics * 0.1.4 2025-05-09 [1] RSPM (R 4.5.0)
#> GenomeInfoDb * 1.44.3 2025-09-21 [1] Bioconductor 3.21 (R 4.5.1)
#> GenomeInfoDbData 1.2.14 2025-05-24 [1] Bioconductor
#> GenomicAlignments 1.44.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> GenomicRanges * 1.60.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> glue 1.8.0 2024-09-30 [2] RSPM (R 4.5.0)
#> htmltools 0.5.8.1 2024-04-04 [2] RSPM (R 4.5.0)
#> htmlwidgets 1.6.4 2023-12-06 [2] RSPM (R 4.5.0)
#> httr 1.4.7 2023-08-15 [1] RSPM (R 4.5.0)
#> IRanges * 2.42.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> jquerylib 0.1.4 2021-04-26 [2] RSPM (R 4.5.0)
#> jsonlite 2.0.0 2025-03-27 [2] RSPM (R 4.5.0)
#> knitcitations * 1.0.12 2021-01-10 [1] RSPM (R 4.5.0)
#> knitr 1.50 2025-03-16 [2] RSPM (R 4.5.0)
#> lattice 0.22-7 2025-04-02 [3] CRAN (R 4.5.1)
#> lifecycle 1.0.4 2023-11-07 [2] RSPM (R 4.5.0)
#> lobstr 1.1.2 2022-06-22 [1] RSPM (R 4.5.0)
#> lubridate 1.9.4 2024-12-08 [1] RSPM (R 4.5.0)
#> magrittr 2.0.4 2025-09-12 [2] RSPM (R 4.5.0)
#> Matrix 1.7-4 2025-08-28 [3] RSPM (R 4.5.0)
#> MatrixGenerics * 1.20.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> matrixStats * 1.5.0 2025-01-07 [1] RSPM (R 4.5.0)
#> memoise 2.0.1 2021-11-26 [2] RSPM (R 4.5.0)
#> pillar 1.11.1 2025-09-17 [2] RSPM (R 4.5.0)
#> pkgconfig 2.0.3 2019-09-22 [2] RSPM (R 4.5.0)
#> pkgdown 2.1.3 2025-05-25 [2] RSPM (R 4.5.0)
#> plyr 1.8.9 2023-10-02 [1] RSPM (R 4.5.0)
#> prettyunits 1.2.0 2023-09-24 [2] RSPM (R 4.5.0)
#> pryr 0.1.6 2023-01-17 [1] RSPM (R 4.5.0)
#> purrr 1.1.0 2025-07-10 [2] RSPM (R 4.5.0)
#> R.methodsS3 1.8.2 2022-06-13 [1] RSPM (R 4.5.0)
#> R.oo 1.27.1 2025-05-02 [1] RSPM (R 4.5.0)
#> R.utils 2.13.0 2025-02-24 [1] RSPM (R 4.5.0)
#> R6 2.6.1 2025-02-15 [2] RSPM (R 4.5.0)
#> ragg 1.5.0 2025-09-02 [2] RSPM (R 4.5.0)
#> Rcpp 1.1.0 2025-07-02 [2] RSPM (R 4.5.0)
#> RCurl 1.98-1.17 2025-03-22 [1] RSPM (R 4.5.0)
#> recount3 * 1.19.3 2025-09-24 [1] Bioconductor
#> RefManageR 1.4.0 2022-09-30 [1] RSPM (R 4.5.0)
#> restfulr 0.0.16 2025-06-27 [1] RSPM (R 4.5.1)
#> rjson 0.2.23 2024-09-16 [1] RSPM (R 4.5.0)
#> rlang 1.1.6 2025-04-11 [2] RSPM (R 4.5.0)
#> rmarkdown 2.29 2024-11-04 [2] RSPM (R 4.5.0)
#> Rsamtools 2.24.1 2025-09-07 [1] Bioconductor 3.21 (R 4.5.1)
#> RSQLite 2.4.3 2025-08-20 [1] RSPM (R 4.5.0)
#> rtracklayer 1.68.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> S4Arrays 1.8.1 2025-06-01 [1] Bioconductor 3.21 (R 4.5.1)
#> S4Vectors * 0.46.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> sass 0.4.10 2025-04-11 [2] RSPM (R 4.5.0)
#> sessioninfo * 1.2.3 2025-02-05 [2] RSPM (R 4.5.0)
#> SparseArray 1.8.1 2025-07-23 [1] Bioconductor 3.21 (R 4.5.1)
#> stringi 1.8.7 2025-03-27 [2] RSPM (R 4.5.0)
#> stringr 1.5.2 2025-09-08 [2] RSPM (R 4.5.0)
#> SummarizedExperiment * 1.38.1 2025-04-30 [1] Bioconductor 3.21 (R 4.5.0)
#> systemfonts 1.2.3 2025-04-30 [2] RSPM (R 4.5.0)
#> textshaping 1.0.3 2025-09-02 [2] RSPM (R 4.5.0)
#> tibble 3.3.0 2025-06-08 [2] RSPM (R 4.5.0)
#> tidyselect 1.2.1 2024-03-11 [1] RSPM (R 4.5.0)
#> timechange 0.3.0 2024-01-18 [1] RSPM (R 4.5.0)
#> UCSC.utils 1.4.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> vctrs 0.6.5 2023-12-01 [2] RSPM (R 4.5.0)
#> withr 3.0.2 2024-10-28 [2] RSPM (R 4.5.0)
#> xfun 0.53 2025-08-19 [2] RSPM (R 4.5.0)
#> XML 3.99-0.19 2025-08-22 [1] RSPM (R 4.5.0)
#> xml2 1.4.0 2025-08-20 [2] RSPM (R 4.5.0)
#> XVector 0.48.0 2025-04-15 [1] Bioconductor 3.21 (R 4.5.0)
#> yaml 2.3.10 2024-07-26 [2] RSPM (R 4.5.0)
#>
#> [1] /__w/_temp/Library
#> [2] /usr/local/lib/R/site-library
#> [3] /usr/local/lib/R/library
#> * ── Packages attached to the search path.
#>
#> ──────────────────────────────────────────────────────────────────────────────────────────────────────────────────────Bibliography
This vignette was generated using BiocStyle (Oleś, 2025) with knitr (Xie, 2014) and rmarkdown (Allaire, Xie, Dervieux, McPherson et al., 2024) running behind the scenes.
Citations made with knitcitations (Boettiger, 2021).
[1] J. Allaire, Y. Xie, C. Dervieux, J. McPherson, et al. rmarkdown: Dynamic Documents for R. R package version 2.29. 2024. https://github.com/rstudio/rmarkdown.
[2] T. Barrett, M. Dowle, A. Srinivasan, J. Gorecki, et
al. data.table: Extension of data.frame. R package
version 1.17.8. 2025. https://r-datatable.com.
[3] D. Bates, M. Maechler, and M. Jagan. Matrix: Sparse and Dense Matrix Classes and Methods. R package version 1.7-4. 2025. https://Matrix.R-forge.R-project.org.
[4] H. Bengtsson. R.utils: Various Programming Utilities. R package version 2.13.0. 2025. https://henrikbengtsson.github.io/R.utils/.
[5] C. Boettiger. knitcitations: Citations for ‘Knitr’ Markdown Files. R package version 1.0.12. 2021. https://github.com/cboettig/knitcitations.
[6] L. Collado-Torres. Explore and download data from the recount3 project. https://github.com/LieberInstitute/recount3 - R package version 1.19.3. 2025. DOI: 10.18129/B9.bioc.recount3. http://www.bioconductor.org/packages/recount3.
[7] L. Collado-Torres, A. Nellore, and A. E. Jaffe. “recount workflow: Accessing over 70,000 human RNA-seq samples with Bioconductor [version 1; referees: 1 approved, 2 approved with reservations]”. In: F1000Research (2017). DOI: 10.12688/f1000research.12223.1. https://f1000research.com/articles/6-1558/v1.
[8] L. Collado-Torres, A. Nellore, K. Kammers, S. E. Ellis, et al. “Reproducible RNA-seq analysis using recount2”. In: Nature Biotechnology (2017). DOI: 10.1038/nbt.3838. http://www.nature.com/nbt/journal/v35/n4/full/nbt.3838.html.
[9] A. C. Frazee, B. Langmead, and J. T. Leek. “ReCount: A multi-experiment resource of analysis-ready RNA-seq gene count datasets”. In: BMC Bioinformatics (2011). DOI: 10.1186/1471-2105-12-449. https://doi.org/10.1186/1471-2105-12-449.
[10] J. Hester. covr: Test Coverage for Packages. R package version 3.6.4. 2023. https://covr.r-lib.org.
[11] W. Huber, V. J. Carey, R. Gentleman, S. Anders, et al. “Orchestrating high-throughput genomic analysis with Bioconductor”. In: Nat Methods (2015). DOI: 10.1038/nmeth.3252.
[12] E. Imada, D. F. Sanchez, L. Collado-Torres, C. Wilks, et al. “Recounting the FANTOM CAGE–Associated Transcriptome”. In: Genome Research (2020). DOI: 10.1101/gr.254656.119. https://doi.org/10.1101/gr.254656.119.
[13] M. Lawrence, R. Gentleman, and V. Carey. “rtracklayer: an R package for interfacing with genome browsers”. In: Bioinformatics 25 (2009), pp. 1841-1842. DOI: 10.1093/bioinformatics/btp328. http://bioinformatics.oxfordjournals.org/content/25/14/1841.abstract.
[14] M. Lawrence, W. Huber, H. Pagès, P. Aboyoun, et al. “Software for Computing and Annotating Genomic Ranges”. In: PLoS Computational Biology 9 (8 2013). DOI: 10.1371/journal.pcbi.1003118. http://www.ploscompbiol.org/article/info%3Adoi%2F10.1371%2Fjournal.pcbi.1003118}.
[15] M. W. McLean. “RefManageR: Import and Manage BibTeX and BibLaTeX References in R”. In: The Journal of Open Source Software (2017). DOI: 10.21105/joss.00338.
[16] M. Morgan, V. Obenchain, J. Hester, and H. Pagès. SummarizedExperiment: SummarizedExperiment container. 2019. DOI: 10.18129/B9.bioc.SummarizedExperiment.
[17] A. Oleś. BiocStyle: Standard styles for vignettes and other Bioconductor documents. R package version 2.36.0. 2025. DOI: 10.18129/B9.bioc.BiocStyle. https://bioconductor.org/packages/BiocStyle.
[18] H. Pagès, M. Lawrence, and P. Aboyoun. S4Vectors: Foundation of vector-like and list-like containers in Bioconductor. R package version 0.46.0. 2025. DOI: 10.18129/B9.bioc.S4Vectors. https://bioconductor.org/packages/S4Vectors.
[19] R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria, 2025. https://www.R-project.org/.
[20] A. Razmara, S. E. Ellis, D. J. Sokolowski, S. Davis, et al. “recount-brain: a curated repository of human brain RNA-seq datasets metadata”. In: bioRxiv (2019). DOI: 10.1101/618025. https://doi.org/10.1101/618025.
[21] L. Shepherd and M. Morgan. BiocFileCache: Manage Files Across Sessions. R package version 2.16.2. 2025. DOI: 10.18129/B9.bioc.BiocFileCache. https://bioconductor.org/packages/BiocFileCache.
[22] H. Wickham. httr: Tools for Working with URLs and HTTP. R package version 1.4.7. 2023. https://httr.r-lib.org/.
[23] H. Wickham. pryr: Tools for Computing on the Language. R package version 0.1.6. 2023. https://github.com/hadley/pryr.
[24] H. Wickham. “testthat: Get Started with Testing”. In: The R Journal 3 (2011), pp. 5-10. https://journal.r-project.org/archive/2011-1/RJournal_2011-1_Wickham.pdf.
[25] H. Wickham, W. Chang, R. Flight, K. Müller, et al. sessioninfo: R Session Information. R package version 1.2.3. 2025. https://github.com/r-lib/sessioninfo#readme.
[26] C. Wilks, S. C. Zheng, F. Y. Chen, R. Charles, et al. “recount3: summaries and queries for large-scale RNA-seq expression and splicing”. In: Genome Biol (2021). DOI: 10.1186/s13059-021-02533-6. https://doi.org/10.1186/s13059-021-02533-6.
[27] Y. Xie. “knitr: A Comprehensive Tool for Reproducible Research in R”. In: Implementing Reproducible Computational Research. Ed. by V. Stodden, F. Leisch and R. D. Peng. ISBN 978-1466561595. Chapman and Hall/CRC, 2014.
GTEx and TCGA are broken up by tissue as described later in this vignette.↩︎
These are 92 control spike-in sequences that are commonly used in bulk RNA-seq projects.↩︎
69 controls sequences.↩︎
This new design allows us to couple the expression data with metadata on the fly, as well as have flexibility in case we uncover an error in the files.↩︎
Although ERCC and SIRV are technically not genes.↩︎
Check the BigWig files section further below.↩︎
For example in recount.bwtool .↩︎


